Table Of Content
- Understanding FDA Requirements for DHF
- How is a DHF Used During and After the Development Process?
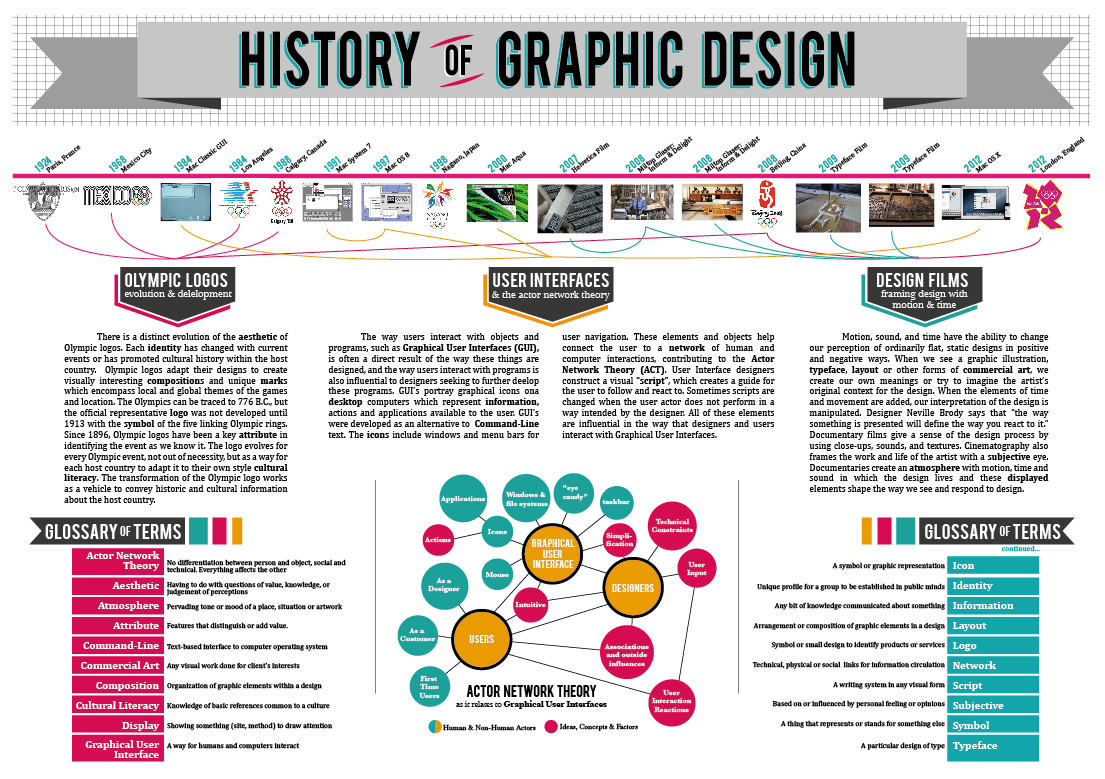
- Debates over the merits of different approaches to teaching design history on practice-based courses
- Staying Ahead of Regulatory Curve with DHF Updates
- Not putting together traceability matrices
- Quality-centric Companies Rely on CQ QMS
- What are the requirements for FDA 21 CFR 820.30 design control compliance?
The DHF allows regulators to observe the development process and assess the potential risks of a device before it reaches market. It’s important to create a strong DHF from the outset, ensure it is regularly updated during development, perform periodic reviews on previously completed projects, and archive old data according to organizational standards. To take proactive steps so your DHF is kept up-to-date and in good shape for future inspections or audits, you must implement design controls.
Understanding FDA Requirements for DHF
However, this guidance does not apply to the software that is not intended for use as a medical device. This document applies to each software device delivered to the end-user, whether factory-installed, or through a third-party vendor, or upgraded version. During the 1980s, the mullet became the standard men's haircut and women sported large, square-cut perms although there were many variations of both. Jumpsuits became a popular element of female clothing and on men, skinny neckties and wraparound sunglasses.
How is a DHF Used During and After the Development Process?
Streamlining the design and approval process is vital to getting products to market in a timely manner. Creating a Design History File (DHF) might be challenging and time-consuming, but there are ways to optimize this process. At HTD, we create DHF in an Agile way and we use the electronic Quality Management System (QMS) from Greenlight Guru. This allows us to significantly accelerate the software as a medical device (SaMD) development process and creation of DHF while staying agile and ensuring compliance with all the regulatory requirements.

Debates over the merits of different approaches to teaching design history on practice-based courses
Salvatore Ferragamo and André Perugia were two of the most influential and respected designers in footwear. Many stars of silent film had a significant impact on fashion during the 1920s, including Louise Brooks, Gloria Swanson, and Colleen Moore. The lighthearted, forward-looking fashions of the 1920s gradually came to halt after the Wall Street Crash of 1929, and succumbed to a more conservative style. While the flapper look persisted into 1930, it quickly disappeared afterward, although bell-shaped hats lasted through 1933. Webinars, training courses, and other resources are available to support organizations in understanding and implementing effective DHF practices. These resources provide guidance, practical examples, and best practices to ensure that the DHF is properly established and maintained throughout the product development process.
Staying Ahead of Regulatory Curve with DHF Updates
Preparing for MDR: Partner Up - MedTech Intelligence
Preparing for MDR: Partner Up.
Posted: Tue, 03 Sep 2019 07:00:00 GMT [source]
Sometimes downstream, the DHF doesn’t represent the product you have on the market, which is not a good situation for the sake of transparency. The advantage to creating traceability at the beginning is visibility over what needs to happen and be addressed. Kudos for having a DHF in the first place as many companies seem to delay getting one together, but having a disorganized file will only create more hassles for you. Many companies make costly and time-consuming errors when creating their DHFs, leading to longer-than-necessary audits and failed FDA inspections. Avoid these mistakes, and you’ll minimize lost time and unnecessary costs getting to market. In the next article, we will delve deeper into how you can create a DHF in an Agile way.
Healthcare - Medical Devices Pharma Digital Health - Tata Elxsi
Healthcare - Medical Devices Pharma Digital Health.
Posted: Mon, 26 Dec 2022 05:57:08 GMT [source]
Streamlining the management of a Design History File (DHF) is crucial in medical device software development. Utilizing advanced document management systems with robust version control and electronic signature capabilities ensures the integrity and security of the DHF. Periodic audits and reviews within a comprehensive quality management system maintain DHF relevance and compliance. Agile methodologies and adaptable DHF architectures accommodate ongoing design modifications and regulatory changes. By addressing these key elements within the DHF, medical device developers can demonstrate regulatory compliance, ensure product quality and safety, and streamline the overall device development process.
Creating the DHF after development is not viable, as it leads to incomplete documentation, regulatory non-compliance, and delays. Real-time documentation ensures accuracy and completeness throughout the design and development process. Collaboration is crucial for DHF efficacy, involving multiple departments and stakeholders.
What are the requirements for FDA 21 CFR 820.30 design control compliance?
And eventually, in 1959, his work culminated in the Empire line, with high-waisted dresses and coats cut similarly to kimono. Balenciaga is also notable as one of the few couturiers in fashion history who could use their own hands to design, cut, and sew the models which symbolized the height of his artistry. Due to difficult times, hemlines crept upward in both evening wear and day wear, the latter of which was made using substitute materials whenever possible. Everyone, from housewives to designers, was forced to re-use of old fabric or create new styles out of old garments. Humor and frivolity became a popstar way of defying the occupying powers and couture survived. Being able to link to and see a final, approved and date stamped ‘issue’ of a document is key to delivering a DHF.
Walter Van Beirendonck, who erupted onto the fashion scene in 1995, produced decidedly futuristic designs under his label W & LT (Wild and Lethal Trash). Deliberately using fabrics developed by the very latest technologies, in violently contrasting colors, he produced clothes that were full of erotic and sadomasochistic references, touched with caustic adolescent humor. As one of the leading online platforms in the medical device sector, 4EasyReg offers extensive support for regulatory compliance. Our services cover a wide range of topics, from EU MDR & IVDR to ISO 13485, encompassing risk management, biocompatibility, usability, software verification and validation, and assistance in preparing technical documentation for MDR compliance. This posts provides an example of organization of the design documentation for a medical device that includes hardware and software.
Jeans remained frayed and bell-bottomed, tie dye was still popular, and the fashion for unisex mushroomed. An immense movement claiming civil rights for blacks combined with the influence of soul music from the USA created a nostalgia for Africa and African culture. A radical chic emerged, influenced by the likes of James Brown, Diana Ross, Angela Davis, and the Black Panthers, in everything from afro hairstyles to platform soles.
As fashion looked to the past, haute couture experienced something of a revival and spawned a myriad of star designers who profited hugely from the rapid growth of the media. The couturier Christian Dior created a tidal wave with his first collection in February 1947. The collection contained dresses with accentuated busts, tiny (or "wasp") waists, and extravagantly full skirts, emphasizing the feminine hourglass figure in a manner very similar to the style of the Belle Époque. The lavish use of fabric and the feminine elegance of the designs appealed to post-war clientele and ensured Dior's meteoric rise to fame.
Throughout the 1950s, although it would be for the last time, women around the world continued to submit to the trends of Parisian haute couture. Three of the most prominent of the Parisian couturiers of the time were Cristóbal Balenciaga, Hubert de Givenchy, and Pierre Balmain. The frugal prince of luxury, Cristóbal Balenciaga Esagri made his fashion debut in the late 1930s. However, it was not until the post-war years that the full scale of the inventiveness of this highly original designer became evident. In 1951, he totally transformed the silhouette, broadening the shoulders and removing the waist. In 1955, he designed the tunic dress, which later developed into the chemise dress of 1957.
Such centralization is critical for maintaining the integrity of the device's history throughout its lifecycle. Configurable item categories and traceability structures allow for customized organization and easy tracking of changes. Supporting agile development methods, the system facilitates faster updates and automated document creation. Enhanced documentation under MDR demands meticulous capture of design rationale and risk management integrations. The TD, equivalent to the FDA's Design History File, becomes even more pivotal under MDR.
It involves meticulous documentation of each design phase, including design inputs, outputs, review, and approval processes, as part of the approved design. Leveraging digital tools for design transfer ensures seamless transition from design to manufacturing, enhancing the traceability and accessibility of design history files. Once a DHF is created, it is essential to keep it up to date, ensuring that all changes to the device design are documented and that the DHF reflects the latest design information.
The DHF ensures that all design activities are properly documented, including design changes and updates. It records the rationale, decision-making process, and impact of design changes, enabling traceability and facilitating effective change management. This documentation is vital for maintaining the integrity of the design and ensuring that design changes are properly evaluated, validated, and implemented. With a career spanning medical device start-ups and fortune 500 companies, Joe has over 25 years of experience in the medical device and high-tech product development industries. A structured document like a Design History File (DHF) is critical to medical device manufacturers.

No comments:
Post a Comment